Optimized Electrodialysis for Wastewater
Optimized Electrodialysis for Wastewater

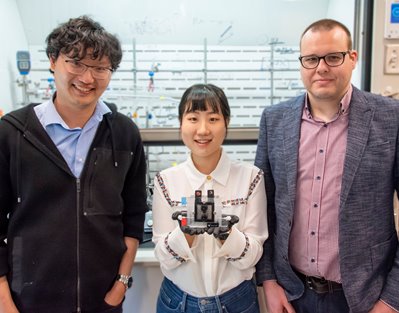
Researchers at the University of Illinois Urbana-Champaign's Beckman Institute have developed a new approach to electrified water purification through desalination.
Desalination removes salt and other minerals from sea water, brackish water, or wastewater but is often expensive and energy intensive. There are several methods to desalinate, including distillation, electrodialysis, and reverse osmosis like the Claude Lewis Plant in Carlsbad, Calif.
In a traditional electrodialysis process, the water is split into positive and negative ions and pulled through ion-exchange membranes leaving the salt behind. However, splitting the water molecules takes a great amount of energy. Membranes are also expensive and foul easily from both the electrodialysis process and organic material in the wastewater. Fouling occurs when particles become stuck on the membranes’ surface and degrade performance. When the ion exchange membranes foul, the resistance for water to pass through them increases, requiring more energy for the process or limiting it all together until the membrane is changed.
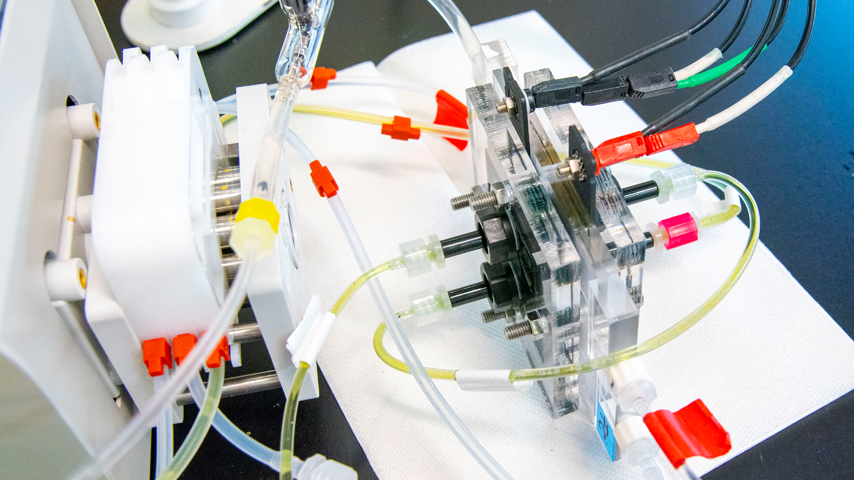
Researchers at the Beckman Institute for Advanced Science and Technology at the University of Illinois Urbana-Champaign have optimized the electrodialysis process by making two significant improvements. First, they used a nanofiltration membrane, made from cellulose, in place of the traditional ion-exchange membrane. The nanofiltration membrane is stable against organic pollutants and does not foul, making it much more robust, explained Xiao Su, a chemical and biomolecular engineering assistant professor and researcher at the Beckman Institute. It is also easier and cheaper to replace since it costs 5 percent to 10 percent of the price of a traditional ion-exchange membrane.
Become a Member: How to Join ASME
Second, the team used a water-soluble polymer in wastewater to alter the electrical charge and attract salt without splitting water molecules. Modelled from the redox flow battery process, which is used in chemical energy storage, the polymer solution reduces energy consumption by 88 percent when compared to traditional electrodialysis.
To demonstrate longevity, long-term stability, and reusability of the polymer, researchers conducted a 70-hour continuous desalination test using a recycled polymer from previous tests. They observed successful and steady desalination throughout the test, stable pH levels, and consistent energy consumption.
In addition to being cheaper and less energy intensive, the optimized electrified dialysis prototype is easily scalable and adaptable to suit the specific needs for the water source, the researchers claim.
“If you have a higher salt concentration, you can increase the voltage and polymer concentration at the click of a button depending on how much you want to desalinate and how much energy you want to use” Su said. During testing, the team increased the voltage from 0.6V to 0.8V and saw an increase in desalination from 69 percent to 119 percent. However, at high operating voltages, side reactions start to occur, making it important to find a balance between desalination and voltage rates.
The prototypes can be installed in a cascading fashion, where the wastewater flows through one segment then the other, each removing a portion of the salt until the water reaches the desired desalination levels. This keeps voltage low within each stage and still achieves the required desalination rates.
“It's very modular. There's a lot of work that can be done to improve the process design, not just the device itself” Su noted. The membranes can also be stacked in an array orientation, like a fan wall, to accommodate a larger volume and flow rate of wastewater.
Additionally, desalination rates can fluctuate with temperature. During testing the room temperature was increased from 5 °C to 21 °C and 60 °C, and achieved 1.19- and 1.33-times higher salt removal, respectively. In geographically favorable locations with hot climates, the prototype can naturally operate at higher temperatures and take advantage of increased desalination rates. There is an opportunity to further optimize the process by pairing the prototype with waste heat from industrial boilers.
You Might Also Enjoy: A New Membrane Slashes the Energy Required to Refine Oil
The specialty polymer was created with ease of manufacturing in mind. Researchers also designed the polymer to be chemically stable, to not cross contaminate water sources, and to have limited side reactions during the process. When treating an equal volume of 100 cubic meters of wastewater, the polymer-nanofiltration membrane prototype costs $0.134 per cubic meter compared to $1.02 per cubic meter for traditional electrodialysis, $2 to $2.6 per cubic meter for distillation, and $0.7 to 1.7 per cubic meter for reverse osmosis.
Su and his team of researchers, including Nayeong Kim and Johannes Elbert, received the Best Paper Award at the Battery Deionization and Electrochemical Separation conference for their paper on the prototype, “Redox-Copolymers for Nanofiltration-Enabled Electrodialysis.”
The team built this prototype with remote communities in mind, hoping to continue developing and testing the prototype for implementation as a small or medium point of source water treatment solution. Due to its low energy consumption, the prototype would pair well with a renewable energy source, such as solar, to further limiting costs and boosting benefits.
Nicole Imeson is an engineer and writer in Calgary, Alberta.

In a traditional electrodialysis process, the water is split into positive and negative ions and pulled through ion-exchange membranes leaving the salt behind. However, splitting the water molecules takes a great amount of energy. Membranes are also expensive and foul easily from both the electrodialysis process and organic material in the wastewater. Fouling occurs when particles become stuck on the membranes’ surface and degrade performance. When the ion exchange membranes foul, the resistance for water to pass through them increases, requiring more energy for the process or limiting it all together until the membrane is changed.
Researchers at the Beckman Institute for Advanced Science and Technology at the University of Illinois Urbana-Champaign have optimized the electrodialysis process by making two significant improvements. First, they used a nanofiltration membrane, made from cellulose, in place of the traditional ion-exchange membrane. The nanofiltration membrane is stable against organic pollutants and does not foul, making it much more robust, explained Xiao Su, a chemical and biomolecular engineering assistant professor and researcher at the Beckman Institute. It is also easier and cheaper to replace since it costs 5 percent to 10 percent of the price of a traditional ion-exchange membrane.
Become a Member: How to Join ASME
Second, the team used a water-soluble polymer in wastewater to alter the electrical charge and attract salt without splitting water molecules. Modelled from the redox flow battery process, which is used in chemical energy storage, the polymer solution reduces energy consumption by 88 percent when compared to traditional electrodialysis.
To demonstrate longevity, long-term stability, and reusability of the polymer, researchers conducted a 70-hour continuous desalination test using a recycled polymer from previous tests. They observed successful and steady desalination throughout the test, stable pH levels, and consistent energy consumption.
A scalable choice
In addition to being cheaper and less energy intensive, the optimized electrified dialysis prototype is easily scalable and adaptable to suit the specific needs for the water source, the researchers claim.
“If you have a higher salt concentration, you can increase the voltage and polymer concentration at the click of a button depending on how much you want to desalinate and how much energy you want to use” Su said. During testing, the team increased the voltage from 0.6V to 0.8V and saw an increase in desalination from 69 percent to 119 percent. However, at high operating voltages, side reactions start to occur, making it important to find a balance between desalination and voltage rates.
The prototypes can be installed in a cascading fashion, where the wastewater flows through one segment then the other, each removing a portion of the salt until the water reaches the desired desalination levels. This keeps voltage low within each stage and still achieves the required desalination rates.
“It's very modular. There's a lot of work that can be done to improve the process design, not just the device itself” Su noted. The membranes can also be stacked in an array orientation, like a fan wall, to accommodate a larger volume and flow rate of wastewater.
Additionally, desalination rates can fluctuate with temperature. During testing the room temperature was increased from 5 °C to 21 °C and 60 °C, and achieved 1.19- and 1.33-times higher salt removal, respectively. In geographically favorable locations with hot climates, the prototype can naturally operate at higher temperatures and take advantage of increased desalination rates. There is an opportunity to further optimize the process by pairing the prototype with waste heat from industrial boilers.
You Might Also Enjoy: A New Membrane Slashes the Energy Required to Refine Oil
The specialty polymer was created with ease of manufacturing in mind. Researchers also designed the polymer to be chemically stable, to not cross contaminate water sources, and to have limited side reactions during the process. When treating an equal volume of 100 cubic meters of wastewater, the polymer-nanofiltration membrane prototype costs $0.134 per cubic meter compared to $1.02 per cubic meter for traditional electrodialysis, $2 to $2.6 per cubic meter for distillation, and $0.7 to 1.7 per cubic meter for reverse osmosis.
Su and his team of researchers, including Nayeong Kim and Johannes Elbert, received the Best Paper Award at the Battery Deionization and Electrochemical Separation conference for their paper on the prototype, “Redox-Copolymers for Nanofiltration-Enabled Electrodialysis.”
The team built this prototype with remote communities in mind, hoping to continue developing and testing the prototype for implementation as a small or medium point of source water treatment solution. Due to its low energy consumption, the prototype would pair well with a renewable energy source, such as solar, to further limiting costs and boosting benefits.
Nicole Imeson is an engineer and writer in Calgary, Alberta.

Pulse of the Profession: Career-Ready Soft Skills
From VR to AI, big data to nanoengineering, engineering advancements are reshaping the mechanical engineering field at a dizzying rate.