Nanoscale Trap Snags Proteins
Nanoscale Trap Snags Proteins
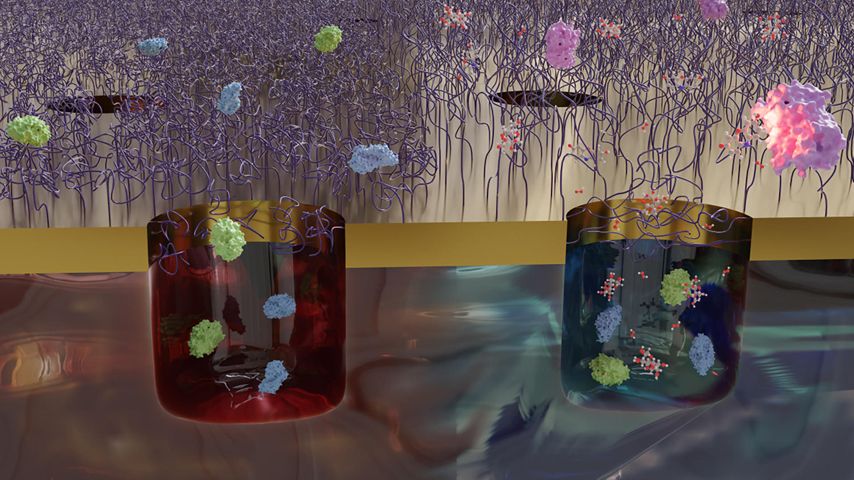

Researchers in Sweden created a way to capture and study proteins. It may assist scientists looking to cure Alzheimer’s and other diseases.
Proteins are the essential building blocks of important bodily tissues and organs. Unfortunately, when protein-related processes go awry in organisms, they can result in proteins misfolding, clumping, or forming unhealthy plaques, ultimately leading to debilitating neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), often referred to as Lou Gehrig’s disease.
While post-mortem studies show researchers the end result of these kind of protein-related problems, there is little known about why proteins may go rogue in different tissues. Now, researchers at Chalmers University of Technology in Sweden have developed a unique nano-sized trap that can capture proteins so scientists can study their interactions in a way that has not been possible before.
Andreas Dahlin, a professor of surface science at Chalmers, works with functional nanostructures, creating synthetic biological systems on the nanoscale for a variety of applications. Nearly 10 years ago, he and his team came up with the idea for a nano-scale gate that could capture proteins and then lock them inside tiny chambers for further study.
“At the beginning, we were just curious if such an idea would work,” he said. “There have been many different methods where researchers have tried to trap these little protein molecules to keep them in place. We decided to try polymers, molecules that consist of long chains, to bring proteins into a nano-scale actuator, capturing them in a small cavity that’s etched out underneath.”
The polymers in this trap work as a sort of gate. They are attached to a special nanoscale chamber treated with special chemicals designed to attract the proteins of interest. At room temperature, those polymers are hydrophilic, stretching out as they interact with water in the system. But when the researchers raise the temperature to 32 °C, the polymers contract, collapsing into the cavity below and lassoing the proteins to bring them into the trap.
“This chamber provides a small volume for the molecules, but we can capture a high concentration of the proteins even in that small space,” said Dahlin. “The polymer keeps them in place as long as the temperature is right. We can then study them as they interact with one another, to better understand what may make them behave in certain ways.”
Once inside the trap, the proteins cannot escape, and the chemistry in the trap allows them to interact with one another in the nano-sized space. Dahlin’s group was able to observe its interactions for up to one hour in its proof-of-concept study, though Dahlin said it is likely it could remain inside this unique system longer under the right conditions.
Editor's Choice: Engineering Proteins to Mine for Rare Earths
This would enable researchers to better understand the different mechanisms, as well as the different sizes and structures of protein folds or clumps, that give rise to different neurodegenerative diseases.
“The protein interactions are what we need to study to understand what may be happening in the beginning of these diseases,” said Dahlin. “This method allows for a very high concentration of the proteins in a small volume so they naturally bump into one another, and we can see what happens.”
While Dahlin said he and his team were pleased that their idea worked, the method requires further refinement to target amyloid-beta, which turns into plaques in Alzheimer’s disease, or alpha-synuclein, the protein that misfolds in aggregates in Parkinson’s disease.
“What proteins we can capture will depend on the charge of the protein,” he explained. “You need the right charge so they can attach to the walls inside the nano-trap cavities. If the walls are charged in one way, you want the proteins to have the opposite charge so they will stick to the walls, and we can capture them with the polymers. In order to study these different proteins, we will have to play around with the chemistry of the interior walls to attract the ones we want. We might even have to change the polymer used to expand and contract to capture the proteins inside the trap.”
Biomedical Advance: Stamp-Like Device Treats Heart Disease, Then Dissolves
This method offers promise to understand how and why different protein-related disease states may begin, but Dahlin said what is most exciting for him is just what is possible using chemistry and nanotechnology.
“It’s pretty cool that you can construct these super tiny gates that open and close when you just press a button to change the temperature,” he said. “It’s quite amazing what you can accomplish on the nanoscale in such tiny systems. We’ve reached amazing precision that would not have been possible just a few years ago. The fact we can now use polymers and nanostructures to create these cool tools for different applications is amazing.”
Kayt Sukel is a technology writer in Houston.
While post-mortem studies show researchers the end result of these kind of protein-related problems, there is little known about why proteins may go rogue in different tissues. Now, researchers at Chalmers University of Technology in Sweden have developed a unique nano-sized trap that can capture proteins so scientists can study their interactions in a way that has not been possible before.
Andreas Dahlin, a professor of surface science at Chalmers, works with functional nanostructures, creating synthetic biological systems on the nanoscale for a variety of applications. Nearly 10 years ago, he and his team came up with the idea for a nano-scale gate that could capture proteins and then lock them inside tiny chambers for further study.
“At the beginning, we were just curious if such an idea would work,” he said. “There have been many different methods where researchers have tried to trap these little protein molecules to keep them in place. We decided to try polymers, molecules that consist of long chains, to bring proteins into a nano-scale actuator, capturing them in a small cavity that’s etched out underneath.”
The polymers in this trap work as a sort of gate. They are attached to a special nanoscale chamber treated with special chemicals designed to attract the proteins of interest. At room temperature, those polymers are hydrophilic, stretching out as they interact with water in the system. But when the researchers raise the temperature to 32 °C, the polymers contract, collapsing into the cavity below and lassoing the proteins to bring them into the trap.
No escape
“This chamber provides a small volume for the molecules, but we can capture a high concentration of the proteins even in that small space,” said Dahlin. “The polymer keeps them in place as long as the temperature is right. We can then study them as they interact with one another, to better understand what may make them behave in certain ways.”
Once inside the trap, the proteins cannot escape, and the chemistry in the trap allows them to interact with one another in the nano-sized space. Dahlin’s group was able to observe its interactions for up to one hour in its proof-of-concept study, though Dahlin said it is likely it could remain inside this unique system longer under the right conditions.
Editor's Choice: Engineering Proteins to Mine for Rare Earths
This would enable researchers to better understand the different mechanisms, as well as the different sizes and structures of protein folds or clumps, that give rise to different neurodegenerative diseases.
“The protein interactions are what we need to study to understand what may be happening in the beginning of these diseases,” said Dahlin. “This method allows for a very high concentration of the proteins in a small volume so they naturally bump into one another, and we can see what happens.”
Targeting amyloid plaques
While Dahlin said he and his team were pleased that their idea worked, the method requires further refinement to target amyloid-beta, which turns into plaques in Alzheimer’s disease, or alpha-synuclein, the protein that misfolds in aggregates in Parkinson’s disease.
“What proteins we can capture will depend on the charge of the protein,” he explained. “You need the right charge so they can attach to the walls inside the nano-trap cavities. If the walls are charged in one way, you want the proteins to have the opposite charge so they will stick to the walls, and we can capture them with the polymers. In order to study these different proteins, we will have to play around with the chemistry of the interior walls to attract the ones we want. We might even have to change the polymer used to expand and contract to capture the proteins inside the trap.”
Biomedical Advance: Stamp-Like Device Treats Heart Disease, Then Dissolves
This method offers promise to understand how and why different protein-related disease states may begin, but Dahlin said what is most exciting for him is just what is possible using chemistry and nanotechnology.
“It’s pretty cool that you can construct these super tiny gates that open and close when you just press a button to change the temperature,” he said. “It’s quite amazing what you can accomplish on the nanoscale in such tiny systems. We’ve reached amazing precision that would not have been possible just a few years ago. The fact we can now use polymers and nanostructures to create these cool tools for different applications is amazing.”
Kayt Sukel is a technology writer in Houston.









