A Robotic Arm at the Microscale
A Robotic Arm at the Microscale
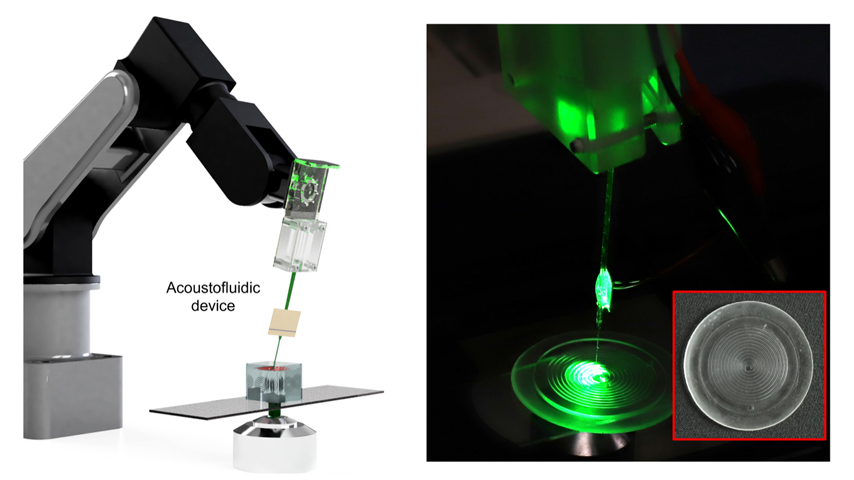

By applying ultrasound at the end of a glass needle, a robotic arm can mix fluid at the micron level.
We owe a lot to the robot arm. So much of today’s welding, painting, cutting, picking, dropping, and, of course, assembly, are done by these automated machines. But it’s at the macro scale where they perform best. When things get smaller than a millimeter, today’s robot arms are too oafish to perform with any precision. As a result, the field of microfluidics has not benefitted from the robotic systems that do so much for other fields.
“If I have a large-scale robotic arm and want to manipulate micron-scale structures, that technology is not there,” said Daniel Ahmed, a professor of acoustic robotics in the Department of Mechanical and Process Engineering at ETH Zurich. “If we can achieve that, then you can manipulate microscale objects in 3D very precisely, and that will open up many applications, starting from manipulation of particles and liquids at microscale to tissue engineering.”
The problem, for the robot—and for microfluidics—is that there are currently no end effectors with enough finesse and agility to mix things at the micron level. Viscosity is stronger at tiny scales, so much so that mixing has not been an option till now.
Ahmed and his team of researchers at ETH Zurich’s Institute of Robotics and Intelligent Systems set out to remedy the issue. Their solution was to mount a piezoelectric transducer onto a thin glass needle, the tip of which is 5 to 10 micrometers in diameter. Their resultant paper, “A robot-assisted acoustofluidic end effector,” appeared in Nature Communications.
When the transducer is turned on, it applies ultrasound to the needle, which starts to oscillate, and create a variety of patterns in whatever medium it’s in—resembling a miniature high-tech spirograph.
Become a Member: How to Join ASME
“You have these really beautiful vortices that are developed along the shaft,” said Ahmed. In essence the needle can draw one fluid into another, atomize it and mix it into a homogenous liquid. The size, shape, and strength of those vortices changes depending on the voltage that is applied. So, in addition to looking pretty, different vortices could be used to mix fluids with different Reynolds numbers.
What’s more, at high amplitudes, the vortices stream around the shaft and create a standing wave there strong enough to trap particles roughly ten microns in size (lower amplitudes can trap larger particles). That means the robotic arm and needle combo could capture single cells. Ahmed and his team used the technique to capture zebrafish embryos.
The advantages of this system for mixing microfluids go beyond the mixing itself. “Typically, if you want to do some microarray experiments, you have to build the device,” says Ahmed. “And the cleanroom facilities always increase the price.”
More for You: Microstructures Improve Design and Function
The robot arm can do more than one task, performing mixing, pumping, and trapping, all simultaneously, and eliminating the need for multiple devices.
Ahmed hopes to further tune the system so that it can inject DNA into a cell—starting with the zebrafish embryo. Eventually it may open up a space where macro and micro movements can happen in the same place.
“Robotic arms are fantastic at automation,” he said. “And microfluidics has many advantages. So we are trying to fuse these things.”
Michal Abrams is a science and technology writer in Westfield, N.J.


microneedle-robot-arm_02.jpg?width=920&height=942&ext=.jpg)





