A Better Pill to Swallow
A Better Pill to Swallow
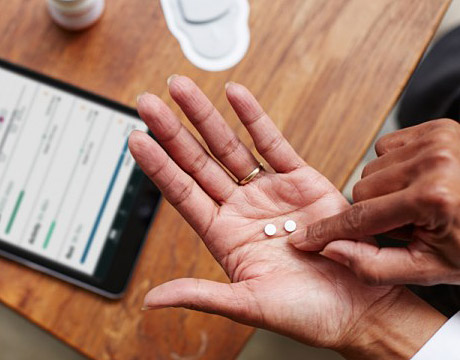

Proteus Discover is a system that icludes smart pills, a patch and a smartphone app. Image: Proteus Digital Health
For millions of Americans who manage chronic health conditions with daily prescription drugs, sticking to the proper dosage instructions can be challenging. A new entry in the wearable sensor technology market could make their doctors’ orders easier to follow – and to swallow.
Medication non-adherence is an expensive and growing problem. Some patients willfully take too little or too much. Some misunderstand the complicated package directions. Others simply forget to take their pills from time to time. Whatever the cause, many of these medication mishaps result in visits to the doctor’s office or emergency room that would have otherwise been unnecessary. As the U.S. population ages and relies on multiple prescriptions to manage complex chronic diseases such as diabetes and dementia, medication mishaps are adding $100 billion to $300 billion to the nation’s annual healthcare bill, according to the U.S. Centers for Disease Control and Prevention (CDC).
The Ingestible Sensor system from Proteus Digital Health (Redwood City, CA) fights this problem from within. Its key feature is a doctor-prescribed Sensor-Enabled Pill patients swallow along with their other medications. Once in the stomach, a non-toxic semiconductor chip within the pill works with externally worn sensors and electronics to create a daily record of medication-taking and physical activity. It’s all part of what Proteus calls a digital health feedback system patients and their doctors can both use to keep chronic diseases in long-term control.
The healthcare industry has long wished for some reliable means of supporting patients who either can’t or won’t follow dosage directions consistently. The Proteus sensor was originally approved as a medical device by the U.S. Food & Drug Administration (FDA) in 2012 and earlier this year earned an expanded indication for use in medication adherence monitoring.
Patient Data
The ingestible sensor – comparable in size to a grain of sand – can be integrated into an inert pill or other edible material, including active pharmaceutical products. The self-powering biosensor is activated by contact with stomach fluids. It identifies what medication the patient has ingested and when, transmitting that data through the patient’s tissues to a detector hidden in an externally worn adhesive patch. The patch also contains devices that record patient activity levels and physiologic data such as heart rate and body position. From there, the data are sent by Bluetooth to a mobile device app patients and their in-home care-givers can use to manage their medication reminders, track their weight and blood pressure, and chart their overall progress. The app connects with a Web-based portal for the patient’s team of professional healthcare providers, who – with patient consent – can remotely follow individual patients’ adherence and treatment response. Data can help inform better doctor-patient communication, identify patients in need of more support, and highlight larger trends among multiple patients.
Proteus manufactures the digestible sensor using a combination of high-volume methods from the semiconductor and pharmaceutical industries. Ingredients are all non-toxic elements present in a normal human diet. Performance-wise, the sensor successfully detects pill ingestion more than 97% of the time and can identify specific pills with 100% accuracy.
Error on Trial
The Proteus device and other ingestible digital health products sure to follow also have promise in pharmaceutical research and development. About half of all Phase III clinical drug trials fail, keeping potentially useful new drugs off the market for reasons not necessarily connected to their actual effectiveness or safety. One frequent cause is a lack of reliable data documenting the trial participants’ adherence to medication protocols. Researchers have previously had to rely on patient self-reporting and lab tests to gauge compliance – often a cumbersome and inaccurate approach.
Oracle Health Sciences (Redwood City, CA) has incorporated Proteus digital feedback data into its InForm Medication Adherence Insights Cloud Service – an electronic data capture and management platform for clinical trials. The system boosts a trial’s chances of success by validating patient adherence data, pinpointing adherence problems early, and helping researchers adjust dosages for improved patient safety or therapeutic effects. At the same time, it reduces trial costs by eliminating time-consuming manual compliance monitoring procedures.
The system addresses “two long-standing and complex challenges: measuring participant adherence to drug protocols and identifying the optimum dosing regimen for recommended use,” said Steve Rosenberg, senior vice president and general manager of Oracle Health Sciences.
Edibles Abound
A number of researchers are developing a taste for edible digital health technologies for applications such as endoscopy, counterfeit drug detection, and physiological status monitoring for military troops stationed in hot climates. At Carnegie Mellon University, for example, engineering professors Christopher Bettinger and Jay Whitacre have teamed up to explore experimental edibles to stimulate damaged tissues, measure digestive health, or deliver drugs to targeted anatomical sites. Their devices combine a shape-memory polymer created in Bettinger’s laboratory with Whitacre’s novel nontoxic sodium-ion battery to provide the minimal amount of power required to activate the structure in the body. The inexpensive battery material passes through the body while the polymeric material remains in place to perform such future applications as gastric monitoring, drug delivery, or tissue repair.
Michael MacRae is an independent writer.
Learn about the latest trends in bioengineering at ASME’s Global Congress onNanoEngineering for Medicine and Biology
Data can help inform better doctor-patient communication, identify patients in need of more support, and highlight larger trends among multiple patients.






.png?width=854&height=480&ext=.png)


