Technique Adds Dimension to Lab-Grown Muscles
Technique Adds Dimension to Lab-Grown Muscles
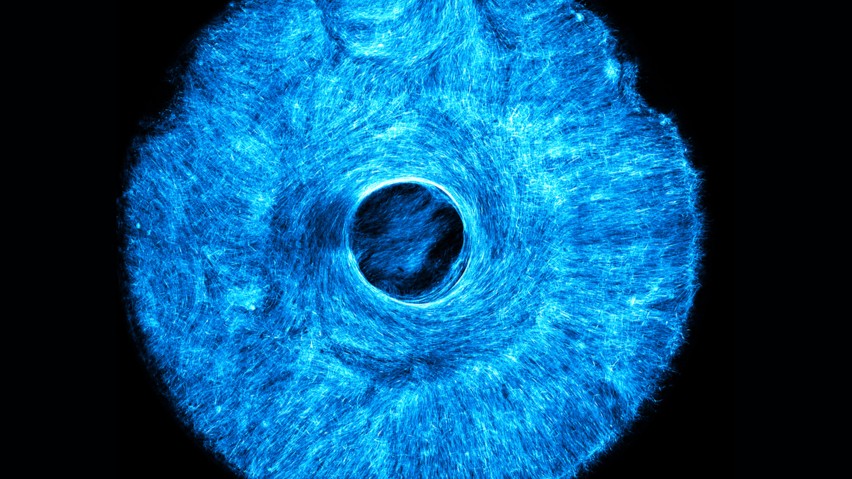

MIT engineers develop way to grow muscles that flex more like natural ones, opening the door to biohybrid robots.
For years, mechanical engineers have experimented with using muscle fibers as potential actuators for “biohybrid” robots—small robots powered by artificially grown muscle fibers. Such bio-bots could wiggle their way into tight spaces that traditional robots cannot enter. So far, researchers have only been able to grow artificial muscle that pulls in one direction, which severely limits any robot’s range of motion.
Now, however, Massachusetts Institute of Technology (MIT) engineers have discovered a way to grow artificial muscle fibers that can flex in multiple coordinated directions. This material pulls both concentrically and radially, which should give biohybrid robots a much greater range of motion.
“We have been working on powering biohybrid robots with living muscle for many years,” said lead researcher Ritu Raman, professor of tissue engineering at MIT’s Department of Mechanical Engineering. “However, in the past, we always focused on building and leveraging uniaxially aligned muscle capable of one-directional motion. Our ‘aha’ moment came from nature—seeing how complex multi-oriented muscle architectures work in nature, which enable multi-degree-of-freedom motion, and wondering if we could replicate these structures in our robots.”
When the researchers stimulated the fibers, the muscle contracted in multiple directions, following the fibers’ orientation—a bioengineering breakthrough that is possibly the first artificially grown muscle tissue that can generate force in more than one direction.
“Muscle tissues operate at the centimeter scale, but each muscle cell is an independent actuator that operates at the micron scale,” said Raman. “Developing a fabrication technique that enabled manufacturing centimeter-scale muscle with precise control over the alignment of single cells was the biggest challenge.”
The stamp can be printed using tabletop 3D printers and fitted with different patterns of microscopic grooves to grow different patterns of muscle tissue. Rather than designing a muscle-powered robot in which all the muscle cells were aligned in one direction, Raman challenged her team to pattern a robot that mimicked the complex multi-oriented architecture of the iris—a central pupil, surrounded by a layer of concentrically aligned muscle and a layer of radially aligned muscle.
“When we stimulated our biohybrid iris with light, the muscle contracted in multiple directions and constricted the pupil, mimicking the way the human eye responds to light stimulation,” said Raman.
Mechanical engineers know how to build machines powered by a wide range of actuators, ranging from pneumatic systems to shape memory alloys. “While these actuators are very useful for some robotic applications, they do not operate at the microscale, and they cannot dynamically adapt to their surroundings in the way nature muscle does,” said Raman. “Our lab is developing methods to predictively design and fabricate robots actuated by muscle, enabling the development of adaptive living machines.”
Going forward, Raman and her colleagues plan to apply the stamping method to other cell types, as well as explore different muscle architectures and ways to activate artificial, multidirectional muscle to do useful work.
“We aim to use our STAMP technique to fabricate increasingly complex biohybrid robots capable of functions such as swimming, pumping, and gripping,” she said. “STAMP enables one-step patterning of micro-topographical cues on hydrogels, which is useful beyond muscle—we are hoping people in other fields will use our tools to build complex tissues composed of other cell types.”
Mark Crawford is a technology writer in Corrales, N.M.
Now, however, Massachusetts Institute of Technology (MIT) engineers have discovered a way to grow artificial muscle fibers that can flex in multiple coordinated directions. This material pulls both concentrically and radially, which should give biohybrid robots a much greater range of motion.
“We have been working on powering biohybrid robots with living muscle for many years,” said lead researcher Ritu Raman, professor of tissue engineering at MIT’s Department of Mechanical Engineering. “However, in the past, we always focused on building and leveraging uniaxially aligned muscle capable of one-directional motion. Our ‘aha’ moment came from nature—seeing how complex multi-oriented muscle architectures work in nature, which enable multi-degree-of-freedom motion, and wondering if we could replicate these structures in our robots.”
Leaving an impression
Raman’s research team grew the artificial muscle tissue using a simple but unique “stamping” approach they developed. The first step was 3D-printing a small, handheld stamp patterned with microscopic grooves, each as small as a single cell. They then pressed the stamp into a soft hydrogel and seeded the resulting grooves with live muscle cells. (Before pressing the stamp into the hydrogel mat, they coated the bottom of the stamp with a protein that helped imprint the pattern evenly into the gel and peel away without sticking or tearing). The cells grew along these grooves within the hydrogel, forming fibers.When the researchers stimulated the fibers, the muscle contracted in multiple directions, following the fibers’ orientation—a bioengineering breakthrough that is possibly the first artificially grown muscle tissue that can generate force in more than one direction.
“Muscle tissues operate at the centimeter scale, but each muscle cell is an independent actuator that operates at the micron scale,” said Raman. “Developing a fabrication technique that enabled manufacturing centimeter-scale muscle with precise control over the alignment of single cells was the biggest challenge.”
The stamp can be printed using tabletop 3D printers and fitted with different patterns of microscopic grooves to grow different patterns of muscle tissue. Rather than designing a muscle-powered robot in which all the muscle cells were aligned in one direction, Raman challenged her team to pattern a robot that mimicked the complex multi-oriented architecture of the iris—a central pupil, surrounded by a layer of concentrically aligned muscle and a layer of radially aligned muscle.
Building an iris
The team printed a stamp pattern similar to the microscopic musculature in the human iris and pressed the pattern into a hydrogel mat, which they had coated with cells that they genetically engineered to respond to light. Within a day, the cells fell into the microscopic grooves and began to fuse into fibers, following the iris-like pattern and eventually growing into a whole muscle, with an architecture and size similar to a real iris.“When we stimulated our biohybrid iris with light, the muscle contracted in multiple directions and constricted the pupil, mimicking the way the human eye responds to light stimulation,” said Raman.
Mechanical engineers know how to build machines powered by a wide range of actuators, ranging from pneumatic systems to shape memory alloys. “While these actuators are very useful for some robotic applications, they do not operate at the microscale, and they cannot dynamically adapt to their surroundings in the way nature muscle does,” said Raman. “Our lab is developing methods to predictively design and fabricate robots actuated by muscle, enabling the development of adaptive living machines.”
Going forward, Raman and her colleagues plan to apply the stamping method to other cell types, as well as explore different muscle architectures and ways to activate artificial, multidirectional muscle to do useful work.
“We aim to use our STAMP technique to fabricate increasingly complex biohybrid robots capable of functions such as swimming, pumping, and gripping,” she said. “STAMP enables one-step patterning of micro-topographical cues on hydrogels, which is useful beyond muscle—we are hoping people in other fields will use our tools to build complex tissues composed of other cell types.”
Mark Crawford is a technology writer in Corrales, N.M.



