Collar Compresses the Neck to Protect the Brain
Collar Compresses the Neck to Protect the Brain
.jpg?width=855&height=480&ext=.jpg)
.jpg?width=855&height=480&ext=.jpg)
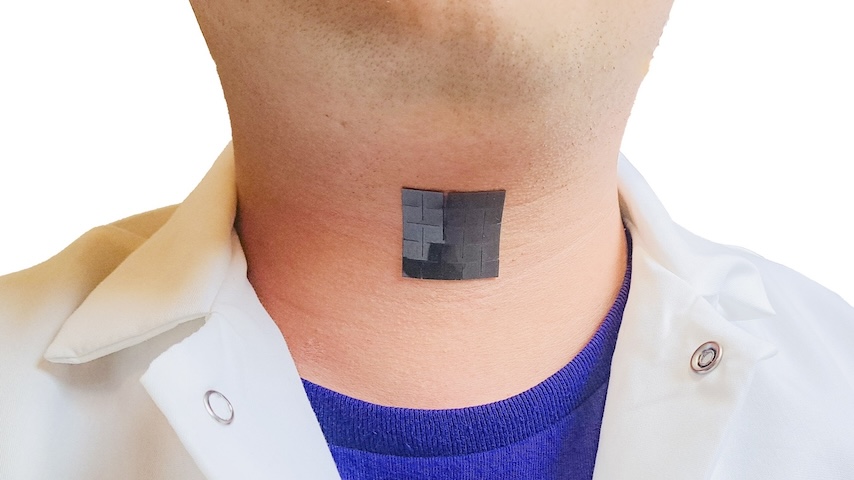
Currently worn by NFL, NCAA, and other athletes who participate in contact sports, the Q-Collar may not prevent concussions, but does provide an additional piece of wearable protective equipment.
The woodpecker hits a tree with its head 20 times a second at 12 times its body weight in force. Yet, its physiology allows it to limit brain motion and avoid injury. This inspiration seemed downright impossible to replicate until early research looked beyond external skull protection and sought an internal approach.
The critical need to protect the brain from trauma led researchers and "the biggest names in brains" to look at jugular vein compression and a simple solution—a device that gently compresses the neck and keeps more blood in the head. Q-Collar was born.
"The Q-Collar intends to protect the brain from injuries caused by head impacts," explained Jamison Float, vice president of innovation, Q30 Innovations, Norwalk, Conn. "Head trauma and repetitive head impacts have short and long-term health consequences for athletes and soldiers." Indeed, the Centers for Disease Control and Prevention and the National Institutes of Health have recently reported that Chronic traumatic encephalopathy (CTE) is likely caused by repeated head traumas. And this brain degeneration is most likely caused in part by repeated hits to the head, called sub-concussive head impacts that happen during contact sports.
Become a Member: How to Join
And while helmets try to protect the skull from the outside, they don't do much for the acceleration and deceleration of the brain inside the skull. Externally worn, the lightweight, horseshoe-shaped Q-Collar seeks to reduce the brain's movement. With the collar in place around the lower portion of the neck, it’s snug and lightly compresses internal jugular veins. This pressure slightly increases the blood volume in the skull's blood vessels. You therefore get less motion during impact because there is now a tighter fit of the brain inside the skull and a reduction of the "slosh" movement.
"The Q-Collar is made from silicone urethane elastomer that encapsulates a highly engineered steel spring that provides a consistent compressive force," Float explained. "It is engineered to apply pressure in the correct area, the sternocleidomastoid muscle in a way that compresses the jugular vein but does it without injuring or interfering with activities."
There are eight different sizes—11 inches up to 18 inches. "The collar will find the place on your neck where it wants to live," Float said, and explained that it is light, and the fit is not too tight to cause discomfort. The compression from the Q-Collar is constant, but there are no reported adverse effects from users wearing the product.
You may be interested: Helmets That Absorb and Respond to Collisions
"There is a little bit more of a break-in period similar to wearing a mouth guard or a helmet for the first time," he explained. “And one of the things we found through all of our clinical trials, and all of our participants and tolerability studies is it usually takes about a week to get used to. And after 3 weeks you start to feel naked without it."
The company was formed in 2012 and the first laboratory research study using a standard acceleration-deceleration model of mild traumatic brain injury found the device prevented axonal injury after internal jugular vein compression. The researchers argued in Neurosurgery Online, "Internal Jugular Vein Compression Mitigates Traumatic Axonal Injury in a Rat Model by Reducing the Intercranial Slosh Effect," that IJV compression worked by increasing intracranial blood volume.
“During the first eight years, we focused on research and development of the Q-Collar, including working with a team of biomedical engineers and our manufacturing partner,” explained Tom Hoey, Q-Collar’s co-founder and current CEO. He described sponsoring more than 25 independent pre-clinical and clinical studies “to demonstrate the safety and effectiveness of the Q-Collar.”
The goal was to get FDA clearance. “We communicated frequently with the FDA as we prepared a comprehensive application, called a De Novo application, which was filed in March 2020,” he explained. Following its review, including a request for additional information, the FDA authorized the Q-Collar for sale as an “over-the-counter” Class II medical device in February 2021. The agency declared that studies showed the device "may reduce the occurrence of specific changes" in the brain associated with brain injury. "By reducing the movement of the brain within the cranial space, the Q-Collar may aid in the protection of the brain from the effects of head impacts," it concluded.
The device cannot eliminate concussions and traumatic brain injuries, Hoey emphasized. "We seek to reduce injury caused by repetitive, sub-concussive impacts that could be what's going to minimize these longer term health conditions whether its dementia, whether its CTE," he said.
In a 2021 press release, Christopher M. Loftus, M.D., acting director of the Office of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health said the Q-Collar "provides an additional piece of protective equipment athletes can wear when playing sports to help protect their brains from the effects of repetitive head impacts while still wearing the personal protective equipment associated with the sport."
According to the FDA, using several studies including a prospective, longitudinal study in the United States involving 284 high school football players, 13 years of age, it assessed the safety and effectiveness of the Q-Collar. During the season, 139 wore the Q-Collar and 145 didn’t. All participants also wore an accelerometer device that measured every impact to the head sustained during play. Each athlete underwent a magnetic resonance imaging (MRI) scan pre-season and post-season. These MRI scans were used to generate Diffusion Tensor Imaging (a specialized MRI image) of the brain that allowed researchers to compare structural changes in the participants’ brain.
"Significant changes were found in deeper tissues of the brain involved in the transmission of electrical nerve signals (white matter regions) in 106 of the 145 (73%) participants in the no-Collar group, while no significant changes in these regions were found in 107 of the 139 (77%) of the group who wore the Q Collar. These differences appear to indicate protection of the brain associated with device use," the FDA reported.
Since 2021, the maker of the Q-Collar has seen athlete acceptance in sports such as bobsleighing, lacrosse, and football. Forty-five NFL players currently wear the device and players at more than a dozen college teams, including Auburn and Alabama, make it part of their sports equipment routine. We are seeing a high rate of adoption "at every level from youth—a lot of interest from high school, a lot of interest from NCAA teams—and a fair bit coming in from the NFL," Float explained.
“Over 500 active service members across all branches are currently wearing the Collar,” Hoey added. And the company recently began work with the U.S. Army via the U.S. Army Medical Materiel Development Activity (USAMMDA) to evaluate if the Q-Collar can help “protect servicemen and women from brain injuries caused by blast waves," he explained.
But despite the Q-Collar being subjected to extensive design validation testing by the FDA to demonstrate durability, reliability, and repeatability in numerous environments, there is always room for improvement, Hoey admitted.
“This fall, we will begin testing a new material for the Q-Collar with our pro athlete partners to gain their feedback with a goal of rolling it out to all consumers in 2024,” he explained. “This material will make the Q-Collar lighter and more comfortable, while maintaining its core functionality. We are also beginning to investigate how biometric tracking could be incorporated into the Q-Collar to allow for the product to help monitor indicators like heart rate.”
Cathy Cecere is a membership content program manager.
The critical need to protect the brain from trauma led researchers and "the biggest names in brains" to look at jugular vein compression and a simple solution—a device that gently compresses the neck and keeps more blood in the head. Q-Collar was born.
How Q-Collar works
"The Q-Collar intends to protect the brain from injuries caused by head impacts," explained Jamison Float, vice president of innovation, Q30 Innovations, Norwalk, Conn. "Head trauma and repetitive head impacts have short and long-term health consequences for athletes and soldiers." Indeed, the Centers for Disease Control and Prevention and the National Institutes of Health have recently reported that Chronic traumatic encephalopathy (CTE) is likely caused by repeated head traumas. And this brain degeneration is most likely caused in part by repeated hits to the head, called sub-concussive head impacts that happen during contact sports.
Become a Member: How to Join
And while helmets try to protect the skull from the outside, they don't do much for the acceleration and deceleration of the brain inside the skull. Externally worn, the lightweight, horseshoe-shaped Q-Collar seeks to reduce the brain's movement. With the collar in place around the lower portion of the neck, it’s snug and lightly compresses internal jugular veins. This pressure slightly increases the blood volume in the skull's blood vessels. You therefore get less motion during impact because there is now a tighter fit of the brain inside the skull and a reduction of the "slosh" movement.
"The Q-Collar is made from silicone urethane elastomer that encapsulates a highly engineered steel spring that provides a consistent compressive force," Float explained. "It is engineered to apply pressure in the correct area, the sternocleidomastoid muscle in a way that compresses the jugular vein but does it without injuring or interfering with activities."
There are eight different sizes—11 inches up to 18 inches. "The collar will find the place on your neck where it wants to live," Float said, and explained that it is light, and the fit is not too tight to cause discomfort. The compression from the Q-Collar is constant, but there are no reported adverse effects from users wearing the product.
You may be interested: Helmets That Absorb and Respond to Collisions
"There is a little bit more of a break-in period similar to wearing a mouth guard or a helmet for the first time," he explained. “And one of the things we found through all of our clinical trials, and all of our participants and tolerability studies is it usually takes about a week to get used to. And after 3 weeks you start to feel naked without it."
Q-Collar data and studies
The company was formed in 2012 and the first laboratory research study using a standard acceleration-deceleration model of mild traumatic brain injury found the device prevented axonal injury after internal jugular vein compression. The researchers argued in Neurosurgery Online, "Internal Jugular Vein Compression Mitigates Traumatic Axonal Injury in a Rat Model by Reducing the Intercranial Slosh Effect," that IJV compression worked by increasing intracranial blood volume.
“During the first eight years, we focused on research and development of the Q-Collar, including working with a team of biomedical engineers and our manufacturing partner,” explained Tom Hoey, Q-Collar’s co-founder and current CEO. He described sponsoring more than 25 independent pre-clinical and clinical studies “to demonstrate the safety and effectiveness of the Q-Collar.”
The goal was to get FDA clearance. “We communicated frequently with the FDA as we prepared a comprehensive application, called a De Novo application, which was filed in March 2020,” he explained. Following its review, including a request for additional information, the FDA authorized the Q-Collar for sale as an “over-the-counter” Class II medical device in February 2021. The agency declared that studies showed the device "may reduce the occurrence of specific changes" in the brain associated with brain injury. "By reducing the movement of the brain within the cranial space, the Q-Collar may aid in the protection of the brain from the effects of head impacts," it concluded.
The device cannot eliminate concussions and traumatic brain injuries, Hoey emphasized. "We seek to reduce injury caused by repetitive, sub-concussive impacts that could be what's going to minimize these longer term health conditions whether its dementia, whether its CTE," he said.
In a 2021 press release, Christopher M. Loftus, M.D., acting director of the Office of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health said the Q-Collar "provides an additional piece of protective equipment athletes can wear when playing sports to help protect their brains from the effects of repetitive head impacts while still wearing the personal protective equipment associated with the sport."
According to the FDA, using several studies including a prospective, longitudinal study in the United States involving 284 high school football players, 13 years of age, it assessed the safety and effectiveness of the Q-Collar. During the season, 139 wore the Q-Collar and 145 didn’t. All participants also wore an accelerometer device that measured every impact to the head sustained during play. Each athlete underwent a magnetic resonance imaging (MRI) scan pre-season and post-season. These MRI scans were used to generate Diffusion Tensor Imaging (a specialized MRI image) of the brain that allowed researchers to compare structural changes in the participants’ brain.
"Significant changes were found in deeper tissues of the brain involved in the transmission of electrical nerve signals (white matter regions) in 106 of the 145 (73%) participants in the no-Collar group, while no significant changes in these regions were found in 107 of the 139 (77%) of the group who wore the Q Collar. These differences appear to indicate protection of the brain associated with device use," the FDA reported.
The future of Q-Collar
Since 2021, the maker of the Q-Collar has seen athlete acceptance in sports such as bobsleighing, lacrosse, and football. Forty-five NFL players currently wear the device and players at more than a dozen college teams, including Auburn and Alabama, make it part of their sports equipment routine. We are seeing a high rate of adoption "at every level from youth—a lot of interest from high school, a lot of interest from NCAA teams—and a fair bit coming in from the NFL," Float explained.
“Over 500 active service members across all branches are currently wearing the Collar,” Hoey added. And the company recently began work with the U.S. Army via the U.S. Army Medical Materiel Development Activity (USAMMDA) to evaluate if the Q-Collar can help “protect servicemen and women from brain injuries caused by blast waves," he explained.
But despite the Q-Collar being subjected to extensive design validation testing by the FDA to demonstrate durability, reliability, and repeatability in numerous environments, there is always room for improvement, Hoey admitted.
“This fall, we will begin testing a new material for the Q-Collar with our pro athlete partners to gain their feedback with a goal of rolling it out to all consumers in 2024,” he explained. “This material will make the Q-Collar lighter and more comfortable, while maintaining its core functionality. We are also beginning to investigate how biometric tracking could be incorporated into the Q-Collar to allow for the product to help monitor indicators like heart rate.”
Cathy Cecere is a membership content program manager.