A Mechanical Biomarker for Brain Health?
A Mechanical Biomarker for Brain Health?
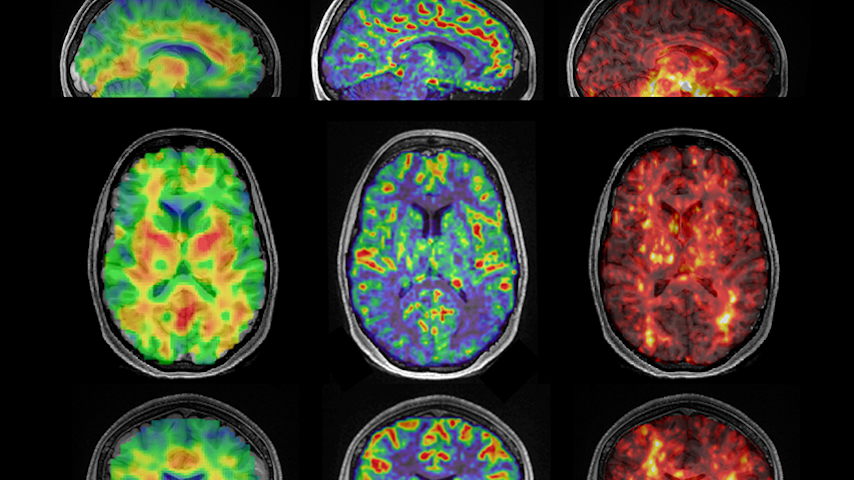

Researchers at the University of Washington have discovered the biomechanical properties of the hippocampus may offer new insights into Alzheimer’s disease.
Deep in the human brain, a small region, delineated by elongated curves, oversees human learning and memory. The hippocampus, so named for its resemblance to a sea horse, has long been implicated in the symptoms seen in Alzheimer’s disease. Neurodegenerative processes appear to attack this area of the brain first, decimating its volume, and leading to the buildup of Alzheimer’s hallmark amyloid beta plaques and tau tangles. That resulting pathophysiology ultimately leads to memory loss, as well as the inability to learn new things or form new memories.
“This is a really interesting region, and it is one of the highest metabolic demand areas in the brain,” said Mehmet Kurt, associate professor of mechanical engineering at the University of Washington. “When you look at its vascular supply, it’s very unique. Its vascular density is low, but it’s very vascularized.”
Because of that uniqueness, many studies have now shown the hippocampus is extremely vulnerable to vascular injury like hypoxia, or lack of oxygen. As it also plays such a vital role in Alzheimer’s disease, Kurt and colleagues wondered if there might be a relationship between perfusion, or blood flow, and the mechanics of hippocampal tissue. It could explain why this area, in particular, is so vulnerable to Alzheimer’s pathology—and complement work done looking at the contributions of amyloid beta and tau.
Kurt’s laboratory, known as KurtLab, studies brain biomechanics, an emerging field that examines how the mechanical properties of tissue might inform development, health, and disease.
“There is a lot of evidence that shows the mechanical microenvironment of tissue is important for forming functional and structural relationships in the brain. Also, the mechanical properties of any tissue is indicative of its health,” he said. “For example, if you had pain in your abdominal area, the first thing your doctor will do is palpate the area, because if something feels abnormal, there is likely an underlying pathology that needs to be addressed.”
Check This Out: It Slices and Dices... Lab Tissue?
The brain is no different. Kurt said that brain biomechanics can be affected by a number of factors, ranging from underlying extracellular matrix properties to neuronal cell density. To gain new insights into how biomechanics might affect hippocampal tissue, he and his team focused on perfusion. The more blood that flows through this structure should inevitably affect its mechanical properties, increasing the stiffness of the surrounding tissue.
“If there is abnormal tissue stiffness in a brain area—let’s say it’s softer—it could be because of cell death,” he said. “But because you have reduced stiffness, it could also create this feedback loop so the tissue keeps getting softer making it harder for blood to flow through it. That would mean the tissue is not getting enough nutrients to supply it which could further soften the tissue and lead to even more cell death like you see in Alzheimer’s.”
To determine the relationship between perfusion and tissue stiffness, Kurt and doctoral student Caitlin Neher scanned the brains of 17 healthy volunteers between the ages of 22 and 35 using a unique technique called magnetic resonance elastography (MRE). MRE is a non-invasive imaging technique that combines traditional magnetic resonance imaging (MRI) with sound waves. As a result, researchers can create detailed measurements of tissue stiffness across the gray matter areas of the brain.
After analyzing the results, the research group discovered that there was a strong association between blood flow and stiffness in the hippocampus. In fact, it was the only region in the brain to show such a relationship. Kurt said the fact it only showed up in the hippocampus—and was as robust as it was—surprised him.
“We actually had two different scanners and two different magnet field strengths. And we saw this in all these different people,” he said. “That was surprising to me.”
Because of the strength of this relationship, Kurt believes, with further research, hippocampal stiffness could be used as a biomarker to help diagnose Alzheimer’s disease in the future.
Discover the Benefits of ASME Membership
“I’m really interested in understanding the cascade of events that lead to Alzheimer’s pathophysiology,” he said. “I believe the hippocampus plays a significant role in that. So, if we can figure out the chicken-and-egg problem here—does tissue softening reduce blood flow leading to depositions of amyloid beta and tau or does reduced blood flow lead to the tissue softening and then those deposits—we could come up with really advanced early diagnostic markers for disease.”
As Kurt continues to investigate these questions, he added that the fact mechanical engineering, as a field, has reinvented itself over the past decade allows researchers like himself to provide innovative new ways to add to our understanding of complex pathophysiology like Alzheimer’s disease.
“We’ve embraced biomedical fields, we’ve embraced machine learning, and we’ve really become a physics-based engineering discipline,” he said. “Using traditional mechanical engineering tools—our MRE method uses simple wave propagation methods to measure stiffness—could make a huge impact in our understanding of human health, as well as other fields.”
Ideally, Kurt would like to follow a cohort of individuals over time to see how hippocampal stiffness might change over time. There’s a lot more to “untangle,” he said, to get to that point. But he is galvanized about what mechanical engineering will add to the discussion about the development and progression of this debilitating disease.
“This is just the beginning,” he said. “There is a unique and very interesting relationship here. So, I’m very excited to see what might come next.”
Kayt Sukel is a technology writer and author in Houston.
“This is a really interesting region, and it is one of the highest metabolic demand areas in the brain,” said Mehmet Kurt, associate professor of mechanical engineering at the University of Washington. “When you look at its vascular supply, it’s very unique. Its vascular density is low, but it’s very vascularized.”
Because of that uniqueness, many studies have now shown the hippocampus is extremely vulnerable to vascular injury like hypoxia, or lack of oxygen. As it also plays such a vital role in Alzheimer’s disease, Kurt and colleagues wondered if there might be a relationship between perfusion, or blood flow, and the mechanics of hippocampal tissue. It could explain why this area, in particular, is so vulnerable to Alzheimer’s pathology—and complement work done looking at the contributions of amyloid beta and tau.
Kurt’s laboratory, known as KurtLab, studies brain biomechanics, an emerging field that examines how the mechanical properties of tissue might inform development, health, and disease.
“There is a lot of evidence that shows the mechanical microenvironment of tissue is important for forming functional and structural relationships in the brain. Also, the mechanical properties of any tissue is indicative of its health,” he said. “For example, if you had pain in your abdominal area, the first thing your doctor will do is palpate the area, because if something feels abnormal, there is likely an underlying pathology that needs to be addressed.”
Check This Out: It Slices and Dices... Lab Tissue?
The brain is no different. Kurt said that brain biomechanics can be affected by a number of factors, ranging from underlying extracellular matrix properties to neuronal cell density. To gain new insights into how biomechanics might affect hippocampal tissue, he and his team focused on perfusion. The more blood that flows through this structure should inevitably affect its mechanical properties, increasing the stiffness of the surrounding tissue.
“If there is abnormal tissue stiffness in a brain area—let’s say it’s softer—it could be because of cell death,” he said. “But because you have reduced stiffness, it could also create this feedback loop so the tissue keeps getting softer making it harder for blood to flow through it. That would mean the tissue is not getting enough nutrients to supply it which could further soften the tissue and lead to even more cell death like you see in Alzheimer’s.”
To determine the relationship between perfusion and tissue stiffness, Kurt and doctoral student Caitlin Neher scanned the brains of 17 healthy volunteers between the ages of 22 and 35 using a unique technique called magnetic resonance elastography (MRE). MRE is a non-invasive imaging technique that combines traditional magnetic resonance imaging (MRI) with sound waves. As a result, researchers can create detailed measurements of tissue stiffness across the gray matter areas of the brain.
After analyzing the results, the research group discovered that there was a strong association between blood flow and stiffness in the hippocampus. In fact, it was the only region in the brain to show such a relationship. Kurt said the fact it only showed up in the hippocampus—and was as robust as it was—surprised him.
“We actually had two different scanners and two different magnet field strengths. And we saw this in all these different people,” he said. “That was surprising to me.”
Because of the strength of this relationship, Kurt believes, with further research, hippocampal stiffness could be used as a biomarker to help diagnose Alzheimer’s disease in the future.
Discover the Benefits of ASME Membership
“I’m really interested in understanding the cascade of events that lead to Alzheimer’s pathophysiology,” he said. “I believe the hippocampus plays a significant role in that. So, if we can figure out the chicken-and-egg problem here—does tissue softening reduce blood flow leading to depositions of amyloid beta and tau or does reduced blood flow lead to the tissue softening and then those deposits—we could come up with really advanced early diagnostic markers for disease.”
As Kurt continues to investigate these questions, he added that the fact mechanical engineering, as a field, has reinvented itself over the past decade allows researchers like himself to provide innovative new ways to add to our understanding of complex pathophysiology like Alzheimer’s disease.
“We’ve embraced biomedical fields, we’ve embraced machine learning, and we’ve really become a physics-based engineering discipline,” he said. “Using traditional mechanical engineering tools—our MRE method uses simple wave propagation methods to measure stiffness—could make a huge impact in our understanding of human health, as well as other fields.”
Ideally, Kurt would like to follow a cohort of individuals over time to see how hippocampal stiffness might change over time. There’s a lot more to “untangle,” he said, to get to that point. But he is galvanized about what mechanical engineering will add to the discussion about the development and progression of this debilitating disease.
“This is just the beginning,” he said. “There is a unique and very interesting relationship here. So, I’m very excited to see what might come next.”
Kayt Sukel is a technology writer and author in Houston.









