Rethinking Medical Device Design in a Post-COVID-19 World
Rethinking Medical Device Design in a Post-COVID-19 World

Learn how the medical device market has changed due to the COVID-19 pandemic and how multiphysics simulation helped foster innovation. Photo: Emphysys
Digital engineering covers several areas of design and manufacturing. Computer-aided design, digital twins, the Internet of Things, and advanced simulation are just a sample of the digital tools available to mechanical engineers today.
COMSOL Multiphysics is a general-purpose simulation software based on advanced numerical methods—applicable to several industries such as electronic design and advanced fluid systems. COMSOL is this month’s sponsor of the Digital Engineering Collection, and the excerpt below is an article published in COMSOL News 2020: The Multiphysics Simulation Magazine.
The introduction of new healthcare technologies has been hampered due to restrictions on trials of medical devices, elective procedures, and access to medical facilities as part of COVID-19 safety protocols. The industry is poised to accelerate the changeover to newer technologies once the dust settles and trials can resume. One example is that device companies are moving toward reversible and irreversible electroporation, where an electrical field is applied to increase the permeability of cell membranes in order to surgically target a tumor or improve drug delivery into the cell. This technique can overcome some of the inherent drawbacks in classical RF ablation but also creates challenges in the power delivery system since higher intensity, shorter pulses of energy are needed.
Technology development companies, such as Emphysys, are helping to meet this challenge. Our scientists and engineers have expertise in multimodal, high-frequency energy generation and software control systems, along with sophisticated simulation abilities to fully understand the system under design. When combined with numerical simulations, this creates a powerful group able to rapidly prototype any energy-based medical device on a much shorter time scale than traditionally expected.
Read Our Blog: Preparing for a Career as a Simulation Engineer in Medical Devicesin Medical Devices
Our company has roots in power generation for plasma sources, devices typically used in the semiconductor manufacturing industry. As such, we have built RF generators ranging from 10 watts to 100 kilowatts, frequencies from DC all the way up to microwave, and waveforms ranging from single and multifrequency sinusoidal to custom pulsed waveforms running in a closed-loop control system.
All of this institutional knowledge lends itself perfectly to the medical device space, where power requirements are usually less than 100 watts, and frequencies are on the order of a few hundred kilohertz. Skilled technology development companies can design custom waveforms in medical devices for electroporation in a way that minimizes unwanted effects, such as neurostimulation and tissue charring. We have developed dedicated physics interfaces within COMSOL Multiphysics to model the electroporation process, as well as the device itself.
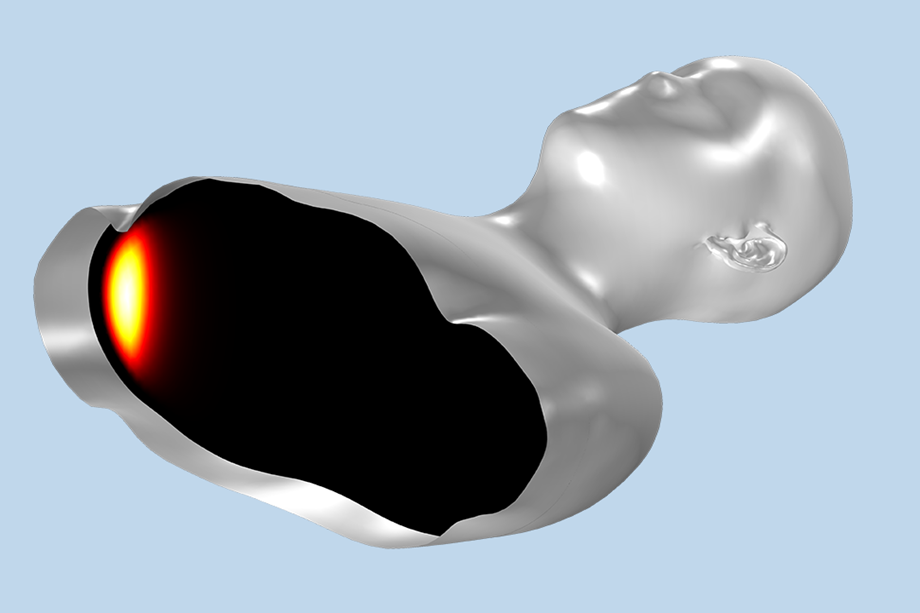
Running electroporation simulations on both a microscopic (of the individual pores) and macroscopic (of the entire handpiece) model allows us to construct a window of operation for the power supply in terms of pulse duration; peak power, current, or voltage; and frequency, in the case that the pulse is an RF signal. This allows us to write a requirement specification for the power supply, which can be constructed in-house by our engineering team. The window provided by the simulations usually means the power supply can meet the needs of the product on the first iteration.
The simulations also allow us to optimize the handpiece, or patient applicator, itself, such that it will work for a wide range of patients’ characteristic impedance since every person is slightly different in terms of weight, muscle density, etc. Once design specifications for the power supply and handpiece are available, the electronics boards; control systems; and software, including the user interface to the device, can be constructed and integrated seamlessly into the system. The fact that we can execute all stages of this process in-house is one of the reasons why we have a competitive edge in terms of the time it takes to go from concept to functional prototype.
Watch Our Video: Bioinspired Breakthroughs Transform Medical Devices
This array of capabilities is also well suited for project recovery. Once medical device trials get back to normal post-COVID-19, many projects will be significantly behind schedule. Emphysys has the expertise of getting such projects back on track in terms of the system design as well as the project timeline. Moving forward, we expect to see a new generation of medical devices based on electroporation as opposed to traditional thermal ablation. Our company philosophy and diverse employee skillset make us uniquely qualified to take advantage of this industry trend in the coming years.
Daniel Smith is the director of modeling and simulation at Emphysys.
COMSOL Multiphysics is a general-purpose simulation software based on advanced numerical methods—applicable to several industries such as electronic design and advanced fluid systems. COMSOL is this month’s sponsor of the Digital Engineering Collection, and the excerpt below is an article published in COMSOL News 2020: The Multiphysics Simulation Magazine.
The introduction of new healthcare technologies has been hampered due to restrictions on trials of medical devices, elective procedures, and access to medical facilities as part of COVID-19 safety protocols. The industry is poised to accelerate the changeover to newer technologies once the dust settles and trials can resume. One example is that device companies are moving toward reversible and irreversible electroporation, where an electrical field is applied to increase the permeability of cell membranes in order to surgically target a tumor or improve drug delivery into the cell. This technique can overcome some of the inherent drawbacks in classical RF ablation but also creates challenges in the power delivery system since higher intensity, shorter pulses of energy are needed.
Technology development companies, such as Emphysys, are helping to meet this challenge. Our scientists and engineers have expertise in multimodal, high-frequency energy generation and software control systems, along with sophisticated simulation abilities to fully understand the system under design. When combined with numerical simulations, this creates a powerful group able to rapidly prototype any energy-based medical device on a much shorter time scale than traditionally expected.
Read Our Blog: Preparing for a Career as a Simulation Engineer in Medical Devicesin Medical Devices
Our company has roots in power generation for plasma sources, devices typically used in the semiconductor manufacturing industry. As such, we have built RF generators ranging from 10 watts to 100 kilowatts, frequencies from DC all the way up to microwave, and waveforms ranging from single and multifrequency sinusoidal to custom pulsed waveforms running in a closed-loop control system.
All of this institutional knowledge lends itself perfectly to the medical device space, where power requirements are usually less than 100 watts, and frequencies are on the order of a few hundred kilohertz. Skilled technology development companies can design custom waveforms in medical devices for electroporation in a way that minimizes unwanted effects, such as neurostimulation and tissue charring. We have developed dedicated physics interfaces within COMSOL Multiphysics to model the electroporation process, as well as the device itself.
Running electroporation simulations on both a microscopic (of the individual pores) and macroscopic (of the entire handpiece) model allows us to construct a window of operation for the power supply in terms of pulse duration; peak power, current, or voltage; and frequency, in the case that the pulse is an RF signal. This allows us to write a requirement specification for the power supply, which can be constructed in-house by our engineering team. The window provided by the simulations usually means the power supply can meet the needs of the product on the first iteration.
The simulations also allow us to optimize the handpiece, or patient applicator, itself, such that it will work for a wide range of patients’ characteristic impedance since every person is slightly different in terms of weight, muscle density, etc. Once design specifications for the power supply and handpiece are available, the electronics boards; control systems; and software, including the user interface to the device, can be constructed and integrated seamlessly into the system. The fact that we can execute all stages of this process in-house is one of the reasons why we have a competitive edge in terms of the time it takes to go from concept to functional prototype.
Watch Our Video: Bioinspired Breakthroughs Transform Medical Devices
This array of capabilities is also well suited for project recovery. Once medical device trials get back to normal post-COVID-19, many projects will be significantly behind schedule. Emphysys has the expertise of getting such projects back on track in terms of the system design as well as the project timeline. Moving forward, we expect to see a new generation of medical devices based on electroporation as opposed to traditional thermal ablation. Our company philosophy and diverse employee skillset make us uniquely qualified to take advantage of this industry trend in the coming years.
Daniel Smith is the director of modeling and simulation at Emphysys.