J&J Places a Big Bet on 3D Printing
J&J Places a Big Bet on 3D Printing


Johnson & Johnson's Neutrogena MaskiD allows consumers to map their face and decide on skin treatments. J&J then 3D prints a customized mask with embedded treatments to fit their face. Photo: J&J
From Band-Aids and Neutrogena beauty products to pharmaceuticals and orthopedic implants, Johnson & Johnson is one of the world’s largest healthcare companies. It is also an emerging leader in additive manufacturing. What makes J&J’s efforts unusual is its desire to use 3D printing to transform all its diverse business segments. In this interview, we discuss how J&J hopes to do this with Sam Onukuri, head and senior fellow of Johnson & Johnson 3D Printing.
Q1: Does J&J think of 3D printing as a strategic technology the same way it thinks of, say, genetics, or artificial intelligence?
Sam Onukuri: Absolutely. If I look at what J&J has invested over the past four or five years, it is clear that we believe it is an important enabler of value to customers and patients. This is true not just for medical devices—there are a lot of models for that—but also for personalized medicine, pharmaceuticals, and consumer products. We see a big value on a global scale across all those businesses.
Q2: What is driving this?
S.O: If you think about digital platforms and solutions, then 3D printing is a physical manifestation of those modalities. There are several areas where it could have an impact. These include personalized products; novel products that take advantage of 3D printing’s design freedom; supply chain production that is efficient, cost-effective, clean, and sustainable; and portability, where we move the point of care closer to the consumer.
Q3: What kind of personalized products can 3D printing help you create?
S.O: In the personalization space, we are either bringing on-demand products closer to our customers or designing things you could never have made the traditional way. So it’s not just personalization, but design freedom and portability that is important.
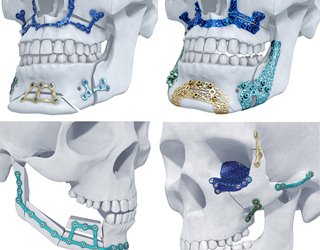
One example is orthopedic products for complex medical procedures. We’re able to create a digital model from a CT or MRI scan and print it out. Surgeons could use the digital model to simulate the surgery and then print patient-specific guides and templates to use during the operation. These are all things that could potentially improve surgical efficiency and clinical outcomes.
Q4: Some hospitals are already printing models like this, right?
S.O: That’s true. In one potential business model, we could work with them to provide new materials or additional services using their existing infrastructure. Or we could possibly partner with a hospital to set up a department to deliver the type of 3D printed products they need. It’s a very flexible business model that’s still evolving and could drive a number of intangibles. For example, hospitals would potentially be able to do more surgeries per day and use customized tools and guides that could reduce patient recovery times.
Recommended for You: 3D Printing Overcoming Biocompatibility Challenge
Q5: You also make personalized implants, right?
S.O: We have commercialized many patient-specific implants for cranio-maxillofacial trauma and spinal surgery, and more recently, large joint reconstruction. We also recently launched a patient-specific bioabsorbable polymer cage for large bone segmental defects caused by cancer.
Q6: Personalization is not just for hospitals. You are also 3D printing consumer products, right?
S.O: Yes, and one example is our Neutrogena MaskiD. There is an app that scans your face, and then you decide what treatments you want on each part of your face. We print a custom mask that fits your anatomy and has those active treatments embedded in it and ship it to you.
This is a technology that could evolve. We could potentially embed sensors in a mask to diagnose what type of cosmetic or even medical care you might need. It could sense pH, cytokines—a small protein secreted by the immune system—and other biometrics. It could be a very effective solution for patients, but that is still years away.
Q7: Could it evolve into something that could treat skin cancer and other skin diseases?
S.O: Right now, it’s strictly for skincare. We remain committed to continuously exploring innovation that will help us to serve patient and customer needs.
Q8: You describe J&J’s additive manufacturing capabilities as a “large ecosystem.” What is it like?
S.O: Our ecosystem includes a lot of internal capabilities, such as multiple material development labs that focus on metals, polymers, biomaterials, bioprinting, and electronics. We also have three end-to-end development lines—in Europe, the United States, and we’re building one in Asia—that can take a solution from the beginning to the end. We also have group additive production capabilities that can print things like implants, instruments, and the like at scale. That gives us a breadth of capabilities across the company. We also collaborate with another 50 external organizations, including academic laboratories, companies, and startups.
Editor's Pick: 3D-Printed Organs Nearing Clinical Trials
It is the combination of all these things that keeps moving the momentum forward. Our group enables the businesses within J&J to build a pipeline of new 3D-printed products by checking the feasibility of applications and bringing them to the businesses to commercialize.
Q9: A lot of companies have turned to additive manufacturing to fight the COVID-19 pandemic. Did J&J ask your additive ecosystem to go beyond its mandate and help?
S.O: Absolutely. Customer needs changed during the pandemic, and 3D printing could provide a lot of value under those circumstances. For example, our Ethicon business is collaborating with the Prisma Health hospital system print and distribute a ventilator expansion splitter that has been authorized for emergency use. We are also participating on a range of collaborative projects, including masks, shields, testing swabs, and things of that nature. We were definitely able to jump in and speed up the delivery of these solutions directly to the customer.
Q10: Additive has so many possibilities. What surprising things do you think we will be doing routinely in five or 10 years?
S.O: One area that attracts me is the bioprinting space. There may come a day when you would go provide the hospital with a sample of your cells, and they would incubate those cells on a 3D-printed scaffold. When you return 30 or 45 days later, there would be a 3D-printed cartilage or meniscus made from your own cells that surgeons could implant in your body. It may be years in the future, but one day this could be a routine surgical process.
Alan S. Brown is senior editor.
Join ASME's AM Medical Virtual Summit on May 27, 2020. Register for FREE!
Q1: Does J&J think of 3D printing as a strategic technology the same way it thinks of, say, genetics, or artificial intelligence?
Sam Onukuri: Absolutely. If I look at what J&J has invested over the past four or five years, it is clear that we believe it is an important enabler of value to customers and patients. This is true not just for medical devices—there are a lot of models for that—but also for personalized medicine, pharmaceuticals, and consumer products. We see a big value on a global scale across all those businesses.
Q2: What is driving this?
S.O: If you think about digital platforms and solutions, then 3D printing is a physical manifestation of those modalities. There are several areas where it could have an impact. These include personalized products; novel products that take advantage of 3D printing’s design freedom; supply chain production that is efficient, cost-effective, clean, and sustainable; and portability, where we move the point of care closer to the consumer.
Q3: What kind of personalized products can 3D printing help you create?
S.O: In the personalization space, we are either bringing on-demand products closer to our customers or designing things you could never have made the traditional way. So it’s not just personalization, but design freedom and portability that is important.
One example is orthopedic products for complex medical procedures. We’re able to create a digital model from a CT or MRI scan and print it out. Surgeons could use the digital model to simulate the surgery and then print patient-specific guides and templates to use during the operation. These are all things that could potentially improve surgical efficiency and clinical outcomes.
Q4: Some hospitals are already printing models like this, right?
S.O: That’s true. In one potential business model, we could work with them to provide new materials or additional services using their existing infrastructure. Or we could possibly partner with a hospital to set up a department to deliver the type of 3D printed products they need. It’s a very flexible business model that’s still evolving and could drive a number of intangibles. For example, hospitals would potentially be able to do more surgeries per day and use customized tools and guides that could reduce patient recovery times.
Recommended for You: 3D Printing Overcoming Biocompatibility Challenge
Q5: You also make personalized implants, right?
S.O: We have commercialized many patient-specific implants for cranio-maxillofacial trauma and spinal surgery, and more recently, large joint reconstruction. We also recently launched a patient-specific bioabsorbable polymer cage for large bone segmental defects caused by cancer.
Q6: Personalization is not just for hospitals. You are also 3D printing consumer products, right?
S.O: Yes, and one example is our Neutrogena MaskiD. There is an app that scans your face, and then you decide what treatments you want on each part of your face. We print a custom mask that fits your anatomy and has those active treatments embedded in it and ship it to you.
This is a technology that could evolve. We could potentially embed sensors in a mask to diagnose what type of cosmetic or even medical care you might need. It could sense pH, cytokines—a small protein secreted by the immune system—and other biometrics. It could be a very effective solution for patients, but that is still years away.
Q7: Could it evolve into something that could treat skin cancer and other skin diseases?
S.O: Right now, it’s strictly for skincare. We remain committed to continuously exploring innovation that will help us to serve patient and customer needs.
Q8: You describe J&J’s additive manufacturing capabilities as a “large ecosystem.” What is it like?
S.O: Our ecosystem includes a lot of internal capabilities, such as multiple material development labs that focus on metals, polymers, biomaterials, bioprinting, and electronics. We also have three end-to-end development lines—in Europe, the United States, and we’re building one in Asia—that can take a solution from the beginning to the end. We also have group additive production capabilities that can print things like implants, instruments, and the like at scale. That gives us a breadth of capabilities across the company. We also collaborate with another 50 external organizations, including academic laboratories, companies, and startups.
Editor's Pick: 3D-Printed Organs Nearing Clinical Trials
It is the combination of all these things that keeps moving the momentum forward. Our group enables the businesses within J&J to build a pipeline of new 3D-printed products by checking the feasibility of applications and bringing them to the businesses to commercialize.
Q9: A lot of companies have turned to additive manufacturing to fight the COVID-19 pandemic. Did J&J ask your additive ecosystem to go beyond its mandate and help?
S.O: Absolutely. Customer needs changed during the pandemic, and 3D printing could provide a lot of value under those circumstances. For example, our Ethicon business is collaborating with the Prisma Health hospital system print and distribute a ventilator expansion splitter that has been authorized for emergency use. We are also participating on a range of collaborative projects, including masks, shields, testing swabs, and things of that nature. We were definitely able to jump in and speed up the delivery of these solutions directly to the customer.
Q10: Additive has so many possibilities. What surprising things do you think we will be doing routinely in five or 10 years?
S.O: One area that attracts me is the bioprinting space. There may come a day when you would go provide the hospital with a sample of your cells, and they would incubate those cells on a 3D-printed scaffold. When you return 30 or 45 days later, there would be a 3D-printed cartilage or meniscus made from your own cells that surgeons could implant in your body. It may be years in the future, but one day this could be a routine surgical process.
Alan S. Brown is senior editor.
Join ASME's AM Medical Virtual Summit on May 27, 2020. Register for FREE!